Introduction
Firstly described in 1967, hyperdiploidy (HD) is the most frequent genetic abnormality in B-cell- precursor acute lymphoblastic leukemia (BCP-ALL) in children, comprising about 25% of all cases. A not yet exactly defined proportion have predisposing pathogenic germline variants in DNA repair pathway genes, chromatin remodeling factors, transcription factors regulating B-cell development (particularly ETV6) or receptor tyrosine kinases pathway genes like RAS/RAF. Among the latter, mutations have mostly been described in PTPN11 and SOS1, but not yet in other components of this central regulatory hub of cellular communication. Germline loss-of-function (LOF) LZTR1 mutations are typically linked to hereditary nerve sheath tumors. However, it remains largely unknown, if other tumor entities are associated with LZTR1 LOF germline mutations, potentially broadening the spectrum of malignancies associated with RASopathies.
Aim
Our aim is to understand the frequency and the impact of LZTR1 germline variants in HD BCP-ALL of childhood.
Material and Methods
We analyzed WES data of 283 children with BCP-ALL for the presence of pathogenic variants in the LZTR1 cancer predisposition gene. For a part of the detected variants, we performed further functional analyses to investigate the effect of the alteration on protein function. We applied a Drosophila model that is particularly suited for functional evaluation of Ras pathway activity.
Results
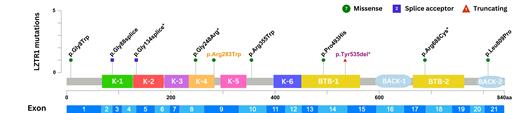
We identified LZTR1 germline variants in 10/283 (3.5%) patients (Figure 1). Interestingly, in 6 out of 283 (2.1%) children the LZTR1 variants were classified as pathogenic/likely pathogenic (P/LP). The majority of patients (5/6) harboring a P/LP germline LZTR1 variant presented with a HD BCP-ALL. Only two of our patients showed concomitant phenotypic features indicative of an underlying syndromic condition. Patient P1 presented with a HD BCP-ALL at the age of 9 years and mild psychomotor delay. Sequencing revealed a likely pathogenic variant (p.Arg283Trp) that was not yet reported in association with RASopathy syndromes. The second child P8, also diagnosed with a HD BCP-ALL, carried a well-known autosomal dominant mutation described in Noonan syndrome (NS) variant (p.Gly248Arg). The child was characterized by facial dysmorphism, as well as mild developmental delay, although a diagnosis of NS was not established prior to development of the BCP-ALL. The other three patients had no clinical peculiarities other than HD BCP-ALL.
We functionally characterized patient derived LZTR1 variants in dLztr1-depleted ISC. Like wild-type hLZTR1 wt, hLZTR1 p.Arg283Trp and hLZTR1 p.Lys761Arg restored control-like ISC lineage production, suggesting that hLZTR1 p.Arg283Trp and hLZTR1 p.Lys761Arg still regulate Ras ubiquitination. In contrast, hLZTR1 p.Tyr535Ter results in even 1.8-fold higher ISC lineage production than dLztr1-RNAi. Beyond ISC production as a readout, we employed a second experimental paradigm directly addressing Ras signalling activity with a translocating modified ERK sensor. In line with Ras activity control of LZTR1, hLZTR1 wt significantly reduced ERK activity, while the putatively LOF variant hLZTR1 p.Tyr535Ter variant significantly increased Ras pathway activation by 1.4-fold over controls. We also noticed that hLZTR1 p.Tyr535Ter induced extensive membrane blebbing and nuclear fragmentation of GFP positive cells likely indicating programmed cell death. Quantification of apoptotic cells revealed an 11.1-fold increase compared to controls, which was not observed for hLZTR1 p.Arg283Trp and hLZTR1 p.Lys761Arg.
Most notably, forced expression of hLZTR1 p.Arg283Trp andhLZTR1 p.Tyr535Ter increased mitotic recombination by 4-fold and 3.4-fold, respectively, which was not observed for the hLZTR1 p. Lys761Arg variant.
Conclusion
In our cohort, approximately 2% of all children with BCP-ALL harboured a P/LP LZTR1 germline variation, not necessarily linked with clinical appearance of Noonan-syndrome-like features, but to the development of a high hyperdiploid karyotype. By applying a Drosophila model, we demonstrated that patient-derived LZTR1 germline variants affect RAS pathway activation, ERK accumulation, cell proliferation, DNA recombination and apoptosis.
Figure 1:
Depiction of the ten LZTR1 variants detected in an unselected pediatric cohort of 283 BCP-ALL patients
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal